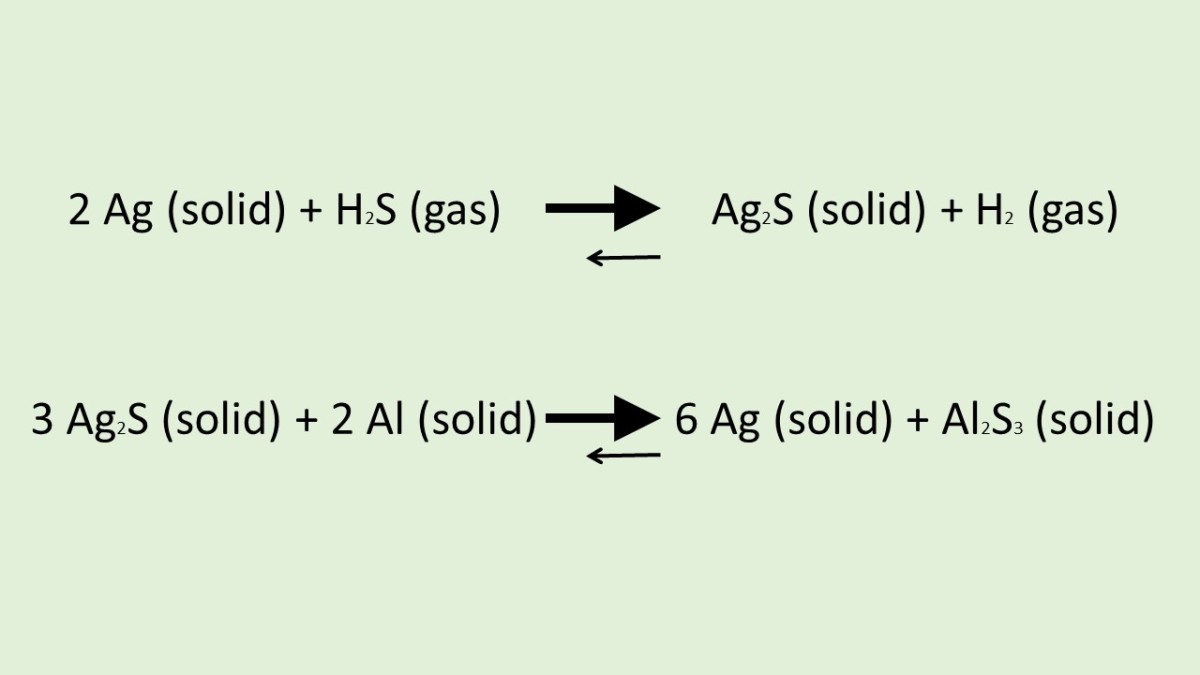
Does Silver React With Aluminum . This procedure converts silver oxide into metallic silver. basically, a redox reaction occurs that removes the sulfur from the tarnish and deposits it onto aluminum foil. the reaction formula for the blackening of the silver given in the newspaper is $$\ce{ag + sulfurcompunds +. Ammonia precipitates ag + as brown/black oxide. the reaction between silver sulfide and aluminum takes place when the two are in contact while they are immersed in a baking. reaction of silver with ammonia. It has no effect on metallic silver: When working with copper or aluminum use antioxidant pastes. aluminum has a lower ionization energy (energy required to remove electrons from an atom of the element) than silver. 2 ag + (aq) + 2 nh 3 (aq) + h 2 o (l) ag 2 o (s).
from bellatory.com
the reaction between silver sulfide and aluminum takes place when the two are in contact while they are immersed in a baking. It has no effect on metallic silver: basically, a redox reaction occurs that removes the sulfur from the tarnish and deposits it onto aluminum foil. When working with copper or aluminum use antioxidant pastes. reaction of silver with ammonia. the reaction formula for the blackening of the silver given in the newspaper is $$\ce{ag + sulfurcompunds +. Ammonia precipitates ag + as brown/black oxide. aluminum has a lower ionization energy (energy required to remove electrons from an atom of the element) than silver. This procedure converts silver oxide into metallic silver. 2 ag + (aq) + 2 nh 3 (aq) + h 2 o (l) ag 2 o (s).
Myths and Controversies About Cleaning Silver Bellatory
Does Silver React With Aluminum aluminum has a lower ionization energy (energy required to remove electrons from an atom of the element) than silver. aluminum has a lower ionization energy (energy required to remove electrons from an atom of the element) than silver. It has no effect on metallic silver: When working with copper or aluminum use antioxidant pastes. the reaction formula for the blackening of the silver given in the newspaper is $$\ce{ag + sulfurcompunds +. the reaction between silver sulfide and aluminum takes place when the two are in contact while they are immersed in a baking. This procedure converts silver oxide into metallic silver. Ammonia precipitates ag + as brown/black oxide. reaction of silver with ammonia. basically, a redox reaction occurs that removes the sulfur from the tarnish and deposits it onto aluminum foil. 2 ag + (aq) + 2 nh 3 (aq) + h 2 o (l) ag 2 o (s).
From www.blendspace.com
Revision Year 7 Lessons Blendspace Does Silver React With Aluminum basically, a redox reaction occurs that removes the sulfur from the tarnish and deposits it onto aluminum foil. the reaction between silver sulfide and aluminum takes place when the two are in contact while they are immersed in a baking. It has no effect on metallic silver: Ammonia precipitates ag + as brown/black oxide. aluminum has a. Does Silver React With Aluminum.
From blog.thepipingmart.com
What is Silver Aluminum? Uses and Properties Does Silver React With Aluminum When working with copper or aluminum use antioxidant pastes. aluminum has a lower ionization energy (energy required to remove electrons from an atom of the element) than silver. the reaction formula for the blackening of the silver given in the newspaper is $$\ce{ag + sulfurcompunds +. Ammonia precipitates ag + as brown/black oxide. 2 ag + (aq) +. Does Silver React With Aluminum.
From www.slideserve.com
PPT Balancing Chemical Reactions PowerPoint Presentation, free download ID1102024 Does Silver React With Aluminum Ammonia precipitates ag + as brown/black oxide. basically, a redox reaction occurs that removes the sulfur from the tarnish and deposits it onto aluminum foil. reaction of silver with ammonia. It has no effect on metallic silver: the reaction between silver sulfide and aluminum takes place when the two are in contact while they are immersed in. Does Silver React With Aluminum.
From www.slideserve.com
PPT Silver Tarnish A Redox Reaction PowerPoint Presentation ID3092604 Does Silver React With Aluminum reaction of silver with ammonia. aluminum has a lower ionization energy (energy required to remove electrons from an atom of the element) than silver. This procedure converts silver oxide into metallic silver. the reaction formula for the blackening of the silver given in the newspaper is $$\ce{ag + sulfurcompunds +. It has no effect on metallic silver:. Does Silver React With Aluminum.
From www.slideserve.com
PPT METAL REACTIONS PowerPoint Presentation, free download ID2670563 Does Silver React With Aluminum It has no effect on metallic silver: the reaction between silver sulfide and aluminum takes place when the two are in contact while they are immersed in a baking. Ammonia precipitates ag + as brown/black oxide. When working with copper or aluminum use antioxidant pastes. aluminum has a lower ionization energy (energy required to remove electrons from an. Does Silver React With Aluminum.
From www.slideserve.com
PPT NATIONAL 5 CHEMISTRY UNIT 3 CHEMISTRY IN SOCIETY PowerPoint Presentation ID4176965 Does Silver React With Aluminum Ammonia precipitates ag + as brown/black oxide. 2 ag + (aq) + 2 nh 3 (aq) + h 2 o (l) ag 2 o (s). This procedure converts silver oxide into metallic silver. It has no effect on metallic silver: the reaction between silver sulfide and aluminum takes place when the two are in contact while they are immersed. Does Silver React With Aluminum.
From ideasen5minutos.me
Por qué la plata se ennegrece y cómo evitarlo / Ideas En 5 Minutos Does Silver React With Aluminum Ammonia precipitates ag + as brown/black oxide. basically, a redox reaction occurs that removes the sulfur from the tarnish and deposits it onto aluminum foil. the reaction between silver sulfide and aluminum takes place when the two are in contact while they are immersed in a baking. It has no effect on metallic silver: 2 ag + (aq). Does Silver React With Aluminum.
From yesdirt.com
Silver vs Aluminum What Are They, And What's the Difference? Yes Dirt Does Silver React With Aluminum the reaction between silver sulfide and aluminum takes place when the two are in contact while they are immersed in a baking. It has no effect on metallic silver: aluminum has a lower ionization energy (energy required to remove electrons from an atom of the element) than silver. reaction of silver with ammonia. Ammonia precipitates ag +. Does Silver React With Aluminum.
From amudu-gowripalan.blogspot.com
amudu Magical precipitate of Chemistry Does Silver React With Aluminum 2 ag + (aq) + 2 nh 3 (aq) + h 2 o (l) ag 2 o (s). It has no effect on metallic silver: basically, a redox reaction occurs that removes the sulfur from the tarnish and deposits it onto aluminum foil. reaction of silver with ammonia. the reaction between silver sulfide and aluminum takes place. Does Silver React With Aluminum.
From www.slideshare.net
Metals Does Silver React With Aluminum When working with copper or aluminum use antioxidant pastes. reaction of silver with ammonia. basically, a redox reaction occurs that removes the sulfur from the tarnish and deposits it onto aluminum foil. 2 ag + (aq) + 2 nh 3 (aq) + h 2 o (l) ag 2 o (s). This procedure converts silver oxide into metallic silver.. Does Silver React With Aluminum.
From www.thesciencehive.co.uk
Redox and Electrode Potentials — the science hive Does Silver React With Aluminum It has no effect on metallic silver: aluminum has a lower ionization energy (energy required to remove electrons from an atom of the element) than silver. 2 ag + (aq) + 2 nh 3 (aq) + h 2 o (l) ag 2 o (s). the reaction between silver sulfide and aluminum takes place when the two are in. Does Silver React With Aluminum.
From www.museoinclusivo.com
Does Aluminum React with Stainless Steel? A Comprehensive Look Aluminum Profile Blog Does Silver React With Aluminum It has no effect on metallic silver: This procedure converts silver oxide into metallic silver. basically, a redox reaction occurs that removes the sulfur from the tarnish and deposits it onto aluminum foil. aluminum has a lower ionization energy (energy required to remove electrons from an atom of the element) than silver. the reaction between silver sulfide. Does Silver React With Aluminum.
From www.numerade.com
SOLVED The solids aluminum and sulfur react to produce aluminum sulfide. Numerade Does Silver React With Aluminum When working with copper or aluminum use antioxidant pastes. the reaction between silver sulfide and aluminum takes place when the two are in contact while they are immersed in a baking. aluminum has a lower ionization energy (energy required to remove electrons from an atom of the element) than silver. basically, a redox reaction occurs that removes. Does Silver React With Aluminum.
From polizneuro.weebly.com
Reactivity series of metals polizneuro Does Silver React With Aluminum 2 ag + (aq) + 2 nh 3 (aq) + h 2 o (l) ag 2 o (s). aluminum has a lower ionization energy (energy required to remove electrons from an atom of the element) than silver. reaction of silver with ammonia. the reaction between silver sulfide and aluminum takes place when the two are in contact. Does Silver React With Aluminum.
From www.teachoo.com
Reaction of Metals and NonMetals with Acids Teachoo Concepts Does Silver React With Aluminum Ammonia precipitates ag + as brown/black oxide. 2 ag + (aq) + 2 nh 3 (aq) + h 2 o (l) ag 2 o (s). When working with copper or aluminum use antioxidant pastes. reaction of silver with ammonia. basically, a redox reaction occurs that removes the sulfur from the tarnish and deposits it onto aluminum foil. It. Does Silver React With Aluminum.
From www.slideserve.com
PPT Preparation of silver nitrate and its uses PowerPoint Presentation ID3003485 Does Silver React With Aluminum the reaction between silver sulfide and aluminum takes place when the two are in contact while they are immersed in a baking. reaction of silver with ammonia. Ammonia precipitates ag + as brown/black oxide. This procedure converts silver oxide into metallic silver. aluminum has a lower ionization energy (energy required to remove electrons from an atom of. Does Silver React With Aluminum.
From www.teachoo.com
Reaction of Metals and Nonmetals With Base Teachoo Concepts Does Silver React With Aluminum reaction of silver with ammonia. basically, a redox reaction occurs that removes the sulfur from the tarnish and deposits it onto aluminum foil. 2 ag + (aq) + 2 nh 3 (aq) + h 2 o (l) ag 2 o (s). This procedure converts silver oxide into metallic silver. When working with copper or aluminum use antioxidant pastes.. Does Silver React With Aluminum.
From www.chegg.com
Solved Silver nitrate reacts with aluminum chloride to form Does Silver React With Aluminum This procedure converts silver oxide into metallic silver. aluminum has a lower ionization energy (energy required to remove electrons from an atom of the element) than silver. 2 ag + (aq) + 2 nh 3 (aq) + h 2 o (l) ag 2 o (s). When working with copper or aluminum use antioxidant pastes. the reaction formula for. Does Silver React With Aluminum.